Ocean Acidification
This lesson plan encourages active learning, scientific inquiry, and environmental stewardship. Understand ocean acidification and its Impact.
-
Description text goes here
-
Description text goes here
-
Description text goes here
Materials Needed:
Whiteboard or chart paper for brainstorming.
Supplies for a demonstration (e.g., clear containers, water, vinegar, baking soda, shells or chalk).
Lesson Structure
1. Introduction
Engage: “Why do we need healthy oceans? What do they do for us?”
Ocean acidification:
The process where excess carbon dioxide (CO₂) in the atmosphere dissolves into the ocean, lowering the pH of seawater.
Human activity such as burning fossil fuels from car emissions or factories and changing deforestation increases atmospheric CO2, which in turn, also increases the amount of carbon dioxide absorbed by the ocean.
The ocean is the largest carbon sink and absorbs 25% of all carbon dioxide.
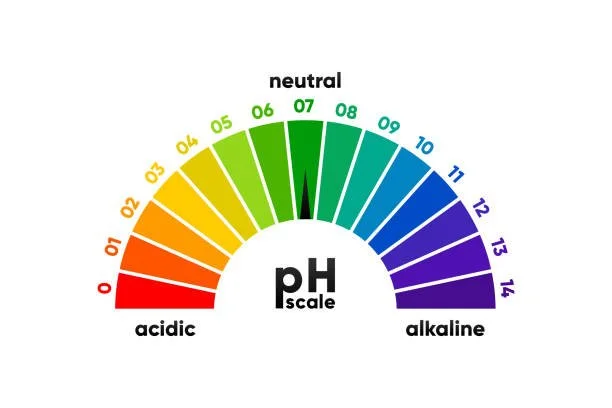
pH scale:
The pH scale ranges from 0-14
0-6.9 is considered acidic; 7.1-14 is considered basic.
A pH of 7 is considered neutral. The further away from 7, the more intense the pH.
The average pH of oceans today is 8.1. Every 0.1 decrease in pH is a ten-fold increase in acidity.
Causes:
CO₂ emissions from fossil fuels- that includes vehicles, factories, power plants, etc.
Deforestation reducing CO₂ absorption by trees.
Chemical Process: CO₂ (carbon dioxide) + H₂O (water) → H₂CO₃ (carbonic acid) → H⁺ + HCO₃⁻ (bicarbonate) → H⁺ + CO₃⁻ (carbonate ions)
H⁺ decreases the pH scale of the ocean and makes it more acidic
Impacts on Marine Life:
Eats away at the minerals needed for organisms (e.g., clams, corals) to form shells and skeletons— animals need carbonate to form shells, but increased H⁺ decreases the availability of carbonate.
Disruption of marine food webs: Animals higher up the food chain that rely on these shell organisms lack food.
Coral bleaching: coral turns white due to loss of symbiotic algae
Reef degradation.
2. Hands-On Demonstration
Experiment Setup:
Fill two clear containers with water.
Add a few drops of vinegar to one container (representing increased CO₂ and acid in seawater).
Place a shell or piece of chalk in both containers.
Observe what happens over time.
Ask students to predict and describe their observations.
Discussion Prompt: How does this relate to marine life like coral reefs and shellfish?
Reflection and Discussion
Discuss:
Why should humans care about ocean acidification?
How can individuals help prevent ocean acidification?
How might it affect people reliant on marine resources?
Reflection Activity: Students should write a short response to: “Imagine you’re a marine animal affected by ocean acidification. Describe your experience and how humans can help.”
4. Assessment
Formative: Participation in the experiment, group activity, and class discussion.
Summative:
Write a paragraph summarizing the causes and effects of ocean acidification.
Create an infographic explaining one solution to reduce its impact.
Enrichment (optional)
Field Study: Visit a local aquarium or marine conservation center to learn about efforts to protect marine life.
Short Video: Effect on Coral Reefs | CBS New Learn